Resources
Geology
This guide by Tim Palmer of the Palaeontological Association will help you to identify the actual stone of the monuments. This can be very helpful when you are thinking about craft workshops and patronage.
Stone is the popular and collective term for all the solid constituents of the Earth’s crust with the exception of ice. Geologists would use the term rock; however the term stone will generally continue to be used here. Rock is a natural mixture of minerals; for example granites consist of a mixture of three different minerals. Minerals are naturally occurring part of the Earth’s crust which consist of one single chemical element or (more usually) several elements combined (compounds); most are a specific crystalline form. Limestones, for example, are composed primarily of the mineral calcite, a form of calcium carbonate. A Crystal is a solid with a regular internal structure consisting of strict arrangement of the smallest particles – molecules, atoms or ions.
Rocks form in the following ways:
- Igneous Rocks form directly from the cooling of magma, the molten material from the centre of the Earth. Igneous rocks include granite and basalt.
- Sedimentary Rocks form from the destruction of rock on the Earth’s surface and subsequent reformation of new rocks from this broken down material. Such rocks include limestone, sandstone and alabaster.
- Metamorphic Rocks are formed when rocks are subject to high temperature or pressure in the deeper parts of the Earth and consequently change their characteristics. Marble and slate are such rocks.
Geological times -when these rocks were formed -are divided and sub-dived into various sections. One such division are periods.
The Jurassic Period mentioned below is from 208 to 146 million years ago. Incidentallywe are still living in the Quaternary Period, which began two million years ago.
Below are a series of illustrated articles by geologist Dr Tim Palmer on the various stones. Click on the bookmark below to be taken to the relevant article.
Oolitic Limestone | Carrara and Purbeck: A Tale of Two Marbles | Magnesium Limestone | Sandstone | Caen Stone
Oolitic Limestone
Many of the medieval monuments in the more southerly counties of England are made of pale, easily carvable limestone of Jurassic age. The detailed geological character of these stones may point to both provenance and date of the monument, so an accurate petrological description is important. The first thing to determine is whether they are oolitic or non-oolitic limestones. This distinction causes much confusion, because some people think that the term oolitic limestone covers all limestones from the Jurassic limestone belt. In fact, it refers only to the specific geological character of some of them.
The great majority of the limestones that we meet in monuments or buildings started as shallow seafloor sediments, consisting of clean-washed sand-sized grains of calcium carbonate. Either these grains were derived from the breakdown of shells and other calcareous skeletons, or else they grew on the seafloor by precipitation of the mineral calcite around smaller particles, just as lime-scale may grow on the inside of a kettle. This second category constitutes the ooliths. Surprisingly, they have a rather limited size range (typically from 0.2-0.8 mm in diameter: when they reached the maximum size they abraded away as fast as they grew and enlarged no further). They are also characteristically very well rounded or spherical. When sliced through and viewed microscopically, the layered structure of the precipitated calcite around the central nucleus is seen. They look like the many layers within the gobstoppers that some of us remember from our youth.
Many Jurassic limestones consist of little other than ooliths, maybe with the oddfossil or fragment. Others have much more shell debris in them, either scattered through a mass of ooliths or (more usually) in debris-rich layers that alternate with oolith-rich layers. This pattern reflects the surging of currents during the initial accumulation of the sediment and is very evident in limestones from near Bath. Other limestones accumulated on the seafloor in areas where ooliths did not form, so the resulting limestones only contain shell fragments from the invertebrate fauna that originally lived there. Geologists refer to these as bioclastic limestones.
Because the smooth spherical character, size-range, and abundance of ooliths is highly distinctive, there should be no problem in recognising them on a cut or rubbed stone surface, especially with a magnifier (no-one should ever venture out without one).Even the concentric laminations and the nucleus may be clear on close inspection. Experience will soon allow distinction between an oolitic limestone and a limestone that is principally composed of comminuted shell debris.
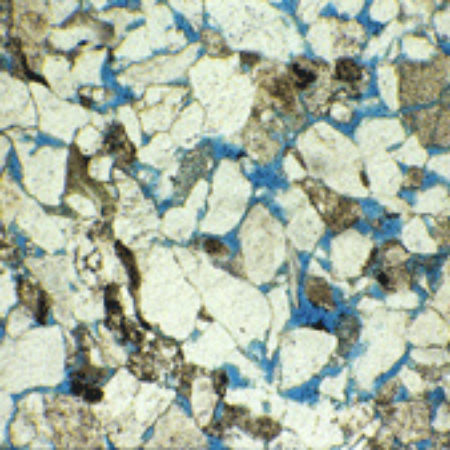
However, there is a further complication that may confuse neophytes and cause one oolitic limestone to look rather different from another. This is the disposition of the natural mineral cement within the stone. Limestones acquire their hardness by growth, during burial, of calcite crystals in the minute holes (pore space) between the grains. This material is popularly called ‘spar’. Some oolitic limestones (e.g.Ketton and Portland) are held together by tiny dabs of spar, principally at the points where adjacent ooliths touch. This is quite enough to give the stone rigidity, and a close look will show the ooliths themselves looking like ball-bearings in a box with the spar not evident (A). But other oolitic limestones (e.g. Bath, B) have their original porespace completely filled with spar, so that the ooliths at the cut surface of the stone may fall away to reveal a surface texture dominated by the concave moulds in the enclosing spar – like close-packed egg-cups on a tray. The first type is referred to as a grain-prominent oolitic limestone, and the second as a spar-prominent one.Yet others have lots of spar, but the individual ooliths are stronger and tend to remain in their little sparry cups at the cut surface. Painswick stone (C) from north of Stroud frequently shows this character.
Each frame (below) is c. 2 cm wide and shows what you would expect to see with a x10 magnifier and a clean surface.
Why, then, are some of the most distinctive non-oolitic limestones that contain only shell debris (Doulting and the highly-important mediaeval Dundry limestone (D) come to mind) persistently and erroneously referred to as oolitic when they are nothing of the sort? Geologists are not completely blameless, because the major Jurassic limestone sequences that run south-west to north-east across England were, in the 19th century, referred to as the Lower, Middle, and Upper Oolites. The Lower was the most widespread and economically important, comprising the Great Oolite of the Bath region, and the Inferior (= subjacent) Oolite near Cheltenham. These stratigraphic units contain many different varieties of limestone of which only some are oolitic,but the name (maybe because it is such a euphonious word) was applied to the whole sequence. Then the historians of architectural materials came along and found it convenient to refer to this swathe of country as the Oolite Belt, and the trap was sprung. Ergo an oolitic limestone is any limestone from the Oolite Belt. Except,of course, that it isn’t.
Photos
 A. Portland Stone with grain-prominent, small ooliths.
A. Portland Stone with grain-prominent, small ooliths. B. Bath Stone with spar-prominent texture.
B. Bath Stone with spar-prominent texture.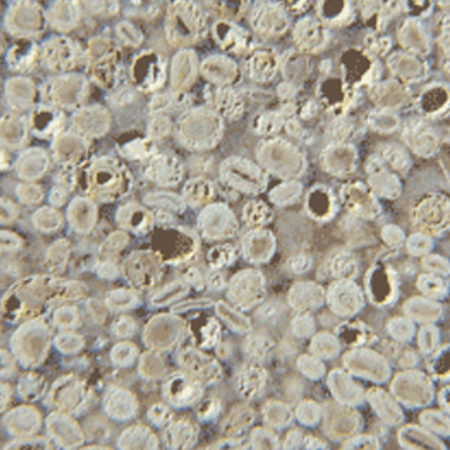 C. Painswick Stone with most ooliths held tightly by the natural cement.
C. Painswick Stone with most ooliths held tightly by the natural cement.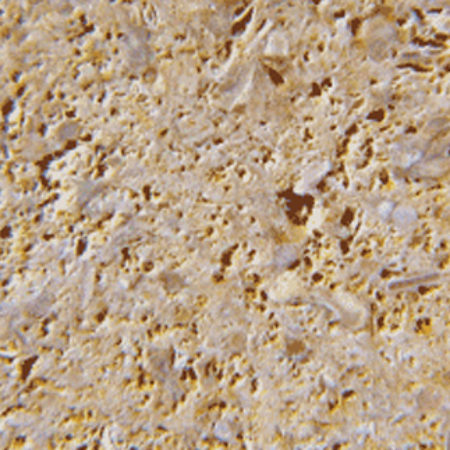 D. Dundry Stone with no ooliths, only shell fragments.
D. Dundry Stone with no ooliths, only shell fragments.
Carrara and Purbeck: A Tale of Two Marbles
It was while I was putting out the rubbish that it occurred to me how I might lead into an explanation of marble as a material. Here in west Wales the plastic wheelie bin does not yet rule supreme, and we are still allowed to use the traditional dustbin.The galvanised surface is a thin layer of zinc that has been induced to grow on the iron vessel by electrolysis. The zinc is in the form of crystals that grew outwards along the iron surface until they met their advancing neighbours and stopped. The result, looked at closely, resembles a jigsaw in which the pieces have angular contacts that abut against each other, rather than interlocking ones. This is the essence of any mass of material that is made up of intergrown crystals. The ice on a frozen windscreen is another – but both ice and zinc galvanisation are two-dimensional crystalline layers. The same thing can also happen in three dimensions, though it is not so familiar to us in our day-to-day experiences. The solid coating of ice that builds up on the walls of an old-fashioned deep-freeze has this structure of intergrowing crystals,
In marble’s case, the material of which the stone is composed is the mineral calcite,the most common of the crystalline varieties of calcium carbonate and one of the 2 or 3 most important minerals in the stones that are used by architects and carvers.The purest white marbles, such as statuary Carrara, are 100% pure calcite. In contrast,most marbles, including other varieties of Carrara, have other minerals (usually mixtures of clays or iron-rich compounds) mixed in with them, and this gives many of them a streaked or grey appearance, or some other colour or pattern. Marked variation in colour and texture is even more evident in the sedimentary marbles, which may also contain fossils. Purbeck is a familiar example.
All marbles started their geological life as limestones, which are also predominantly composed of calcite, and the formation of marble from limestone principally involved a textural change. The original limestone sediment was a mix of grains and limey mud. Shell fragments and ooliths (see the last Newsletter) often featured. Each of these components was itself a jumble of carbonate crystals, of widely differing shapes,sizes, and orientations. In addition, there were originally minute spaces between the constituents. A thought-experiment helps: imagine a bowl of sugar (a crystallinesubstance), assembled as a mixture of all the different varieties that can be found in the kitchen cupboard: granulated and caster; powered icing sugar and large hard lumps of coffee sugar. Air fills the tiny pore-spaces between the grains. If we dampen this mixture, the surfaces of the grains dissolve: on drying, the grains stick together as lumps because the dissolved sugar crystallises and forms a bond where adjacent grains touch. The finest particles may dissolve completely and end up as additionsto the surfaces of the larger crystals. This is the analogy to the growth of a true metamorphic marble like Carrara. In a damp, warm environment buried within the earth,small crystals of calcite go into solution and the atoms (more correctly ions, because they carry an electrical charge) migrate a short distance to the surfaces of large crystals on which they settle and add to the solid crystal structure. Over time,the smallest crystals disappear and larger ones grow at their expense until they meet their neighbours and cannot enlarge further. Typically the average size of the crystals at this stage is 0.5 -1 mm across. Fossils and individual grains in the parent limestone become less distinct as they dissolve and reprecipitate. If there are impurities in the limestone, they may undergo chemical alteration and form darker streaks, wisps and veins. This is the process by which a porous limestone becomes a dense marble of solid interlocking crystals as seen in the Figure below (the similarity to the galvanised texture is obvious though the crystal size is much smaller and the fabric is 3-dimensional). The warmer the burial environment, the faster (geologically speaking) the alteration process – an example of metamorphism – occurs. That is whylimestones that are buried deeply or close to hot volcanic rocks readily become altered to marble. The parent limestone does not have to be very old: the limestones that recrystallised to give Carrara marble were about the same age as the famous fossil-bearing rocks of Lyme Regis.
In contrast, a sedimentary marble like Purbeck has undergone recrystallisation by a rather different process. The famous pond-snails that provide the main constituent of Purbeck Marble lived in a coastal freshwater lagoon, and its floor was composed of a 2 foot deep layer of the dead shells. These shells were made of aragonite, another calcium carbonate mineral that commonly makes up the shells of molluscs. Calcite and aragonite have exactly the same atoms in them in the same proportions, but aragonite is more soluble than calcite in the damp buried environment that persisted as the lake floor became covered by later deposits above it. The aragonite shells started to be replaced by calcite crystals, which seeded and grew outwards at the expense of their more soluble aragonite neighbours. Soon the advancing calcite crystals,as in Carrara, met one another and the replacement of aragonite by a mosaic of calcite crystals was complete. So both types of marble show a fabric of tightly abutting calcite crystals that with little space between them (see the figure below), but the origins or the fabrics are rather different in the two cases. In Purbeck Marble,spaces between the shells also filled up with a natural cement of intergrown calcite crystals so that the whole rock became dense and crystalline. Minor additional iron minerals coloured it blue-grey, red, or green.
When a marble is polished, it is the individual calcite crystals that are cut andsmoothed to a high-gloss finish. Many ordinary limestones can never achieve this because, at a microscopic level, they are full of pores. Only when a limestone loses these can a glossy finish be achieved. Some older limestones (Hopton Wood from the Carboniferous of Derbyshire comes to mind) have gradually lost their pores through natural calcite cement growing in the original pore-spaces, and have achieved enough closely-grown large calcite crystals to become polishable. Anglesey and Egglestone marbles are other examples of Carboniferous hard limestones that will take a polish.
Geologists often get rather stuffy about applying the term marble to the polishable limestones, asserting that the word should only be used for the true metamorphic product. I don’t take this view: after all, the term marble has been applied to polishable limestone since well back into the Middle Ages, whereas the subject (and the name)of Geology only started in the 18th century.
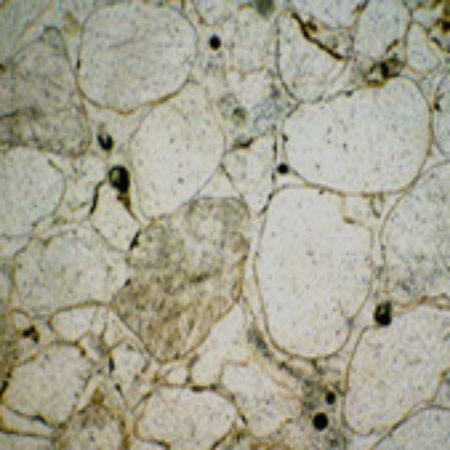
Photographs of polished surfaces of Carrara and Purbeck marbles seen under the electron microscope. In the first specimen the width of the view is about 2mm; in the lower one, about 4mm. In both cases the texture is one of closely intergrown calcite crystals. Purbeck also shows the outlines of the pond-snail shells where the original aragoneite has been replaced by calcite.
Photos
Magnesium Limestone
The geological map of England shows, usually marked in dark blue, a thin belt of stone that runs from Nottingham, northwards past Tadcaster and Ripon, before curling eastwards and meeting the coast in Teeside. This is the outcrop of the Magnesian Limestone, a stone that has been of great importance in the architectural and monumental history of northeast England since Roman times. In the Middle Ages, it provided the free stone used for the great ecclesiastical buildings of the region, such as the minsters at York and Beverley as well as numerous lesser buildings, and countless monuments. For some royal projects it was sent to London (appearing in written records as ‘Northern Stone’). It is still quarried near Tadcaster to meet restoration demands.At the southern end of the outcrop it is used in Southwell Minster, and its magnificence as a building stone there so impressed the commissioners who were recommending the stone for the new Palace of Westminster in 1839, that it was their first choice.A terrible mistake, for much Magnesian Limestone decays most horribly in polluted atmospheres, and within 10 years or so the London sulphur-rich smogs were starting to cause stone-rot and disfigurement.
Magnesian limestone is strong to pale yellow in colour, some varieties being almost white. Though readily worked and carved like the more familiar light-coloured monumental limestones of southern England, it has a different chemical composition and a more complicated geological background. All limestones have essentially a two-stage origin.Usually, the grains are produced on the sea-floor and deposited as beds of sand.Broken shell material and the familiar spherical ooliths are the most abundant grain types, and they are made of calcium carbonate in the form of one or other of two common minerals, calcite or aragonite. Bath Stone, Portland Stone, and the Magnesian Limestone (the lower, stone-bearing part of which is now referred to by geologists as the Cadeby Formation) all started like this, on shallow floors of ancient seas.In the case of the Magnesian Limestone, the sea covered eastern England and extended to central Europe during Permian times, about 250 million years ago. German geologists long ago named this ancient seaway the Zechstein Sea, and this name has stuck among geologists. Its western margin lay against the line of the Pennines and it was on the nearshore shallows that reefs flourished and shoals of oolitic sand accumulated.
This depositional phase of a stone’s history is followed by further alteration when the stone becomes buried below the seafloor (often much deeper) and undergoes diagenesis. The most common diagenetic change is the filling-up of the space between the grains with a crystalline natural cement of calcite, deposited from fluids that circulate through the buried rock. This is the main way in which a sedimentary rock becomes hard. However, circulating water sometimes has a chemical composition that can react with the minerals of the parent sediment, and cause different minerals to precipitate. Limestones are susceptible to such a change if there is a high ratio of magnesium to calcium in the water. Then the calcite of the original sediment is dissolved out and replaced by the mineral dolomite, which is also a carbonate mineral, but which has equal amounts of magnesium and calcium atoms within its crystal structure. This replacement may be partial, so that there are isolated crystals of dolomite in an original calcite matrix, or complete, so that all the calcite is replaced. Dolomite is easy to spot under a microscope because the crystals form minute rhombohedra. In a completely dolomitised limestone, the texture is often sugary, and the original texture of the parent limestone is masked or obliterated.
One geological scenario that leads to dolomitisation in the sediments below the sea-floor,is evaporation of the sea-water, a process that effectively concentrates magnesium.The Zechstein Sea was wide, shallow, and warm; it lay only a few degrees north of the Permian equator. Evaporation was an important process, and this is reflected in the Permian deposits of potash and salt that are mined in the north of England.Fig. 1 shows a sample of magnesian limestone viewed microscopically. There are faint outlines of the original grains, probably ooliths. They have been partially replaced by a mosaic of rhombohedral dolomite crystals that are diamond-shaped in this 2-dimensional slice. There is quite a lot of space in the rock as a result of volume changes that often occur when original calcite is replaced by dolomite. The resulting rock is therefore quite soft and easily workable. Together with the textural and colour uniformityof this type of stone, this is a boon for the carver. In internal use, in masonry and for monuments, magnesian limestone may suffer from some physical abrasion but chemical attack is not usually a problem. However, the high porosities of many dolomitic rocks, and the magnesium-rich mineral composition, combine to produce susceptibility to chemical weathering in polluted external environments where wetting and drying alternate. Rain water is held within the pores by capillarity, and if the water is acidic it slowly dissolves the adjacent dolomite crystals. This was a very great problem before the Clean Air acts of the mid 20th century, when atmospheric water(fog particularly) was a sulphurous acid derived from coal-burning. The acid attack on the dolomite led to formation of magnesium sulphate (better known as Epsom salts).This is a soluble material, and when the stone dried, many crystals of the salt grew in the pores between the dolomite granules, prising them apart as they enlarged.The surface of the stonework weathered back and lost carved detail. Sometimes deep unsightly pits developed, where the white decaying stone would contrast with surrounding soot-blackened areas. In internal monuments, a major problem is dirt, which seems to get deep into the minute pore spaces within the stone and is impossible to clean out by non-destructive methods.
A recent well-illustrated and non-technical book on Stone from the newly-constituted English Stone Forum has a good account of the Magnesian Limestone. The full reference is: Lott, G.K. & Cooper, A.H. Field guide to the Building Limestones of the Upper Permian Cadeby Formation (Magnesian Limestone) of Yorkshire. Pp.80-89 in Doyle, P.(ed.), 2008. England’s Heritage in Stone. English Stone Forum, 111pp.
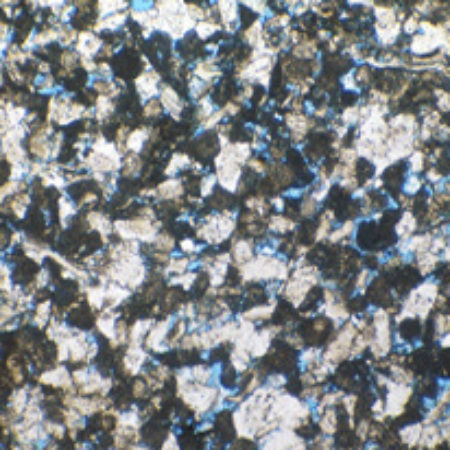
Fig. 1. Microscopic views of thin slices of Magnesian Limestone from Nottinghamshire .1a, general view of fine, sugary dolomite crystals with plenty of intercrystalline space (filled with blue resin in the colour version). The width of view is about 2 mm. 1b, closer view of a specimen with larger dolomite crystals. The original sediment grains of the parent limestone show up as faint outlines within the replacement dolomite crystals; width of view about 0.5 mm.
Photos
 View of fine, sugary dolomite crystals with plenty of intercrystalline space
View of fine, sugary dolomite crystals with plenty of intercrystalline space Closer view of a specimen with larger dolomite crystals
Closer view of a specimen with larger dolomite crystals
Sandstone
The great majority of English medieval monuments are made of limestone (either freestone or polishable marble) or alabaster. However, there is a third type of common sedimentary rock of which monumentalists should have a working knowledge, and that is sandstone.Sandstone buildings and monuments predominate in the part of England and Wales that lies to the north-west of the oolite belt (the strip of country where Jurassic limestones crop out, that runs from Dorset to the Humber). Geological units that yield good quality sandstone freestones include the red sandstones of the West Midlands, Cheshire and the Eden basin; the buff and brown sandstones of Carboniferous age in coal-mining areas and in the millstone grit region of the Peak District and the Pennines; the brown sandstones of the North York Moors (deposited during the Jurassic period, and the age equivalent of the oolites further to the south); and various more local sandstone outcrops in Wales, the Weald, and south-west England. Quality of carving on sandstone memorials in often rather crude and this may be because of their greater hardness when newly quarried, particularly in coarse-grained varieties of the stone. Alternatively,it may point to fewer local schools of carving with experienced masons away from the more fashionable and lucrative limestone or alabaster areas.
Like other sedimentary rocks, sandstones consist of two components: grains that date from their early geological history as a soft sediment, and a cement that grew between the grains after they had been deposited and become buried. The grains were originally transported by water or air, and the sites of deposition were in rivers or deltas,or on the sea-floor, or in dune-fields such as those we are familiar with today from the Sahara desert. The rocks the were eroded to give rise to the individual grains may have been earlier sandstones, or they may have been igneous rocks such as granite.Ultimately, such igneous rocks were the sources of all the individual grains in any sandstone, even if several cycles of erosion, transport, deposition, hardening, and new deposition occurred subsequently. The most durable individual grains were thosemade of quartz (silicon dioxide), the hardest of the minerals that are found in granites which originally cooled from molten magma within the Earth’s crust. Within each episode of transport, the myriads of quartz grains rubbed against and abraded one another and , wearing each other down and becoming smooth and progressively rounded. Transported by wind in deserts (e.g. the New Red Sandstone of Torquay and Penrith) produced better-rounded grains than transported by rivers (e.g. the York stone of the Pennines), and the shape of the individual grains, easily seen with a lend, are a clue to recognising sandstone type. In less mature sandstones (nearer their original igneous source),there may be a mixture of particles derived from other granite materials, such as feldspar or mica. In contrast, mature sandstones contain only quartz; other materials have decomposed to clay and had been winnowed out during the transport phase. A few sandstones of marine origin contain characteristic fossil shells or mineralised invertebrate droppings, tinged green by the mineral glauconite. These are very easy to recognise with a lens, and examples (e.g. malmstone, Gatton stone, Reigate stone) come from the greensand formations that underlie the chalk in the south-eastern counties of England. The suite of De La Beche monuments at Aldworth (Berkshire) contain fourthat are cut from greensand (the other five being very different oolitic limestones),and Reigate stone was of great importance as a freestone in medieval London.
Photos
Thin section photographs of two sandstones. First is a sandstone with very angular grains. The spaces between the grains are empty (filled with blue resin to illustrate this feature in the web version of this article). Second,the grains are well rounded (originally transported by wind and deposited as a dune in an ancient desert). Their rims have a light covering of haematite (red iron oxide), which gives a pinkish tinge to the stone. Width of view = c.2 mm in both.
By far the most conspicuous variable feature in sandstones, however, is the colour– white, pale buff, rusty, dark brown, pink, purplish red, dark tan, blue-grey. Except possibly in the (rare) white case, the colour always lies in the material that grows on, and fills the spaces between, the individual quartz grains. This is the cement that precipitates to fill the pores after the original sediment has become buried.The chemical character of these cements varies with the temperature, the pressure,and the chemical composition of the ground water from which the cement minerals grow.Most contain iron in one form or another. The red and pink cements are haematite: iron oxide that typically precipitated close to the water-table below deserts, which is why the desert sandstones (New Red Sandstone) of the West Midlands and Cheshire are so coloured. The river and delta sandstones of northern England are shades of rusty brown, and their cement (like rust itself) is iron hydroxide. Many of the Welsh Carboniferous sandstones have a cement of clay minerals and are grey and easily eroded;some south-eastern English and southern Welsh sandstones have a calcite cement, like limestones do. A few sandstones show a cement of quartz growing tightly onto the original quartz sediment grains, forming a strong bond between cement and grain,and resulting in a very hard rock that is generally too intractable for memorial carving. The hardest of these are referred to as quartzites. The quartz grains in the haematitic pink sandstones of the West Midlands are often only weakly bound to their neighbours, and the grains are easily dislodged from the sculpted surface by wear and weather. In this way sculpted detail is often removed or lost completely in such sandstones.
Caen Stone
It is becoming clear that the freestones that were used in medieval funerary monuments are largely, and not particularly surprisingly, the same freestones that were used in the fabrics of contemporaneous buildings. Often, a monument that is caked in candle grease and dust in a dark recess within a church, will turn out to be made of the same material that can be seen much more clearly within the structure of the same building. However, this is not invariably so and traps lie hidden for the monumental petrographer. I suspect that one stone that was used more commonly in monuments than is hitherto recognised is Caen Stone from Normandy. It can be obtained in large blocks and it has a uniform, fine-grained texture. It is soft when newly quarried and easy to carve, and it is known to have been extensively imported into southern England, wars permitting, throughout the middle ages, and again in the 19th century. Blocks of the stone (known as gobbets in early texts) would doubtless have been obtainable from merchant importers or from ongoing building projects within southern Britain, and need not necessarily point to a particular connection between Normandy and the commemorating family.
Caen stone is a pure, pale buff limestone of the same geological age as Bath stone.Its texture, however is completely different. Bath stone is made almost entirely of ooliths, quarter to half a millimetre across, whereas Caen stone contains no ooliths whatsoever. It is made of a mixture of the minute plates that once comprised the skeletons of sea-lilies and their zoological relatives (the echinoderms, to the initiated), and the droppings of the same animals. Sea-lilies fed by filtering the sea water in which they lived, and they produced small compacted balls of mud, made up from the tiny suspended inorganic particles that they separated from the useful organic material that provided the food. Such pellets are common in many Jurassic limestones, but of overwhelming abundance in the composition of Caen stone. They are too small to been seen with the naked eye, but appear as cloudy dark blobs, less than 0.1 mm across, under the microscope (see figure).
Photos
Caen stone from the 18th C. fabric of Westminster Abbey, seen under the light microscope. The field of view is about 3.5 mm across. The bright grains are skeletal fragments and the dark rounded pellets are invertebrate droppings. The blue areas in between(seen clearly in the web version of this article) are coloured resin that was used to consolidate the sample while it was being cut and mounted on a glass slide. In this sample, the pellets and the skeletal fragments are present in roughly equal quantities, but other varieties of Caen stone are predominately made up of one or the other grain type (see text).
In architectural work, Caen Stone that was imported in the 19th century often shows terrible decay, whereas the medieval variety still survives in many buildings. This has led to a belief that all the Victorian stone was of poor quality, whereas the early material was uniformly good. In fact, a recent study on the Caen stone at Canterbury Cathedral and in other medieval buildings suggests that a mixture of qualities has always been available. The poor quality variety in the older buildings has usually decayed and has been replaced during the course of the building’s history, often with other types of stone, so that the good quality pieces of Caen stone that remain behind are erroneously interpreted as representing the quality of all of the original material. Good quality and poor quality Caen stone looks quite different when viewed microscopically, because they contain different proportions of the 2 different typesof grain: skeletal fragments and muddy droppings. The former are rigid and attracta dense calcite cement on them during the stone’s geological history. In samples where they predominate, the stone is hard and weathers very slowly. In contrast,samples in which the droppings predominate are softer and more susceptible to wear and weathering. Externally exposed Caen stone shows this contrast most clearly,but it would probably also be discernable in stone that was used internally, as in monuments. The harder variety tends to be somewhat paler in colour and the skeletal grains tend to stand somewhat proud on a worn surface, often looking (rather confusingly) like protuberant creamy ooliths. The softer variety, in contrast, tends towards a yellower colour and wears back where it has been worn by much touching.